Information for Patients
Rhenium-SCT® is to be a painless, personalised, non-invasive therapy targeting and destroying cancer cells.
How does it work?
Rhenium-SCT® utilises the radioactive properties of rhenium-188 in the form of a paste to treat certain non-melanoma skin cancers. The treating clinician precisely applies the paste over a protective film that is affixed to the lesion to be treated. The patient sits comfortably during the painless, single-session treatment for a carefully calculated period of time (usually 30-180 minutes, depending on the size of the lesion).
Rhenium-SCT® works by destroying cancer cells, however, the activity is only minimally penetrative into the skin meaning that underlying healthy tissue is spared.
The paste used in Rhenium-SCT® treatment does not directly touch your skin. You will not be radioactive after treatment and there is no risk to anyone else that you come into contact with.3
What is rhenium-188?
Rhenium-188 is an isotope that emits ß-radiation, which has excellent therapeutic advantages.
ß-radiation is the emission of ß-particles, which can penetrate the human tissue only up to 2-3 mm deep. Therefore, rhenium-188 is ideal in treating superficial tissue without affecting other parts of the body.
It has a half-life of 17 hours, which means that the radioactivity decays up to the half in 17 hours while emitting ß-radiation. This allows treatments to be performed in a very short period of time.

Prior to consideration for
Rhenium-SCT®
Your medical professional will discuss the various options available for the treatment of your NMSC.
Part of the process for determining suitability is a punch or excisional biopsy, which will confirm the tumour type as well as depth.
Tumour depth is important to determine suitability for Rhenium-SCT®, as well as treatment time.
To determine whether treatment with Rhenium-SCT® is right for you, your medical professional will perform a punch biopsy.
This involves taking a small sample of tissue with a small circular blade (called a punch) so that a pathologist can examine it. The procedure is performed under a local anaesthetic and usually takes 10 to 15 minutes.9
A punch biopsy is required to confirm the BCC or SCC diagnosis and to determine how deep the tumour is. Tumours must be less than 3 mm deep to be suitable for Rhenium-SCT®.3
The punch biopsy results and photographs of the lesion will then be included with
your referral.
Following these assessments, you will be referred to a licensed nuclear medicine physician or radiation oncologist. At this appointment, suitability of the tumour for Rhenium-SCT® will be discussed and they will explain what is involved in the procedure so that you can provide your consent.
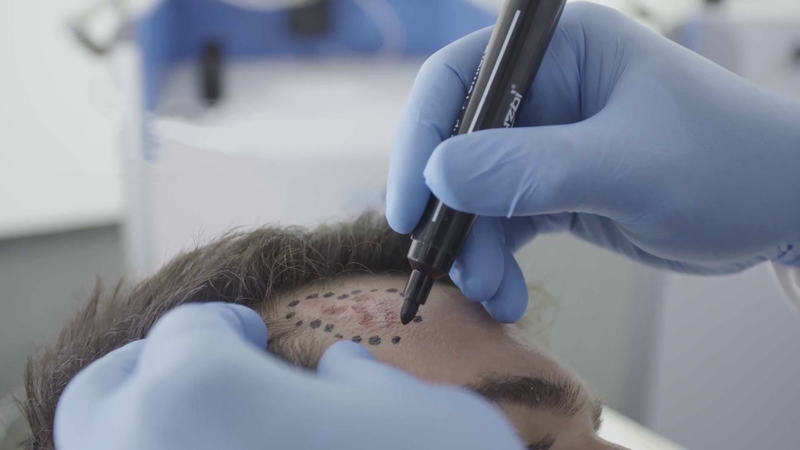
Your medical professional will assess you and mark the treatment area. This will
include the tumour as well as some nearby normal-looking tissue (known as the margin). Including a margin helps ensure that all the cancer cells are removed. The area calculation will be used to calculate your Rhenium-SCT® treatment.
Treatment process
Rhenium-SCT® (Skin Cancer Therapy) is a form of nuclear medicine that utilises the Beta emitter radioisotope rhenium-188 for the treatment of non-melanoma skin cancer.
1
Preparation
The area to be treated is demarcated

2
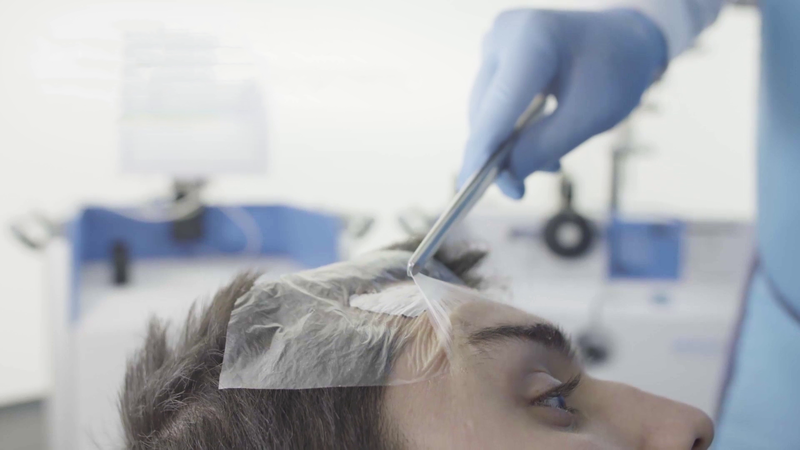
Treatment
The Rhenium-SCT® resin is applied to a protective film affixed to the skin.
3
Treatment Completion
Once treatment time has elapsed, the film is removed. The patient can immediately return to regular activities.
Patient Enquiries
For general enquiries, please complete the contact form and your request will be forwarded to a treatment centre for follow up.
Healthcare Professional?
If you are a healthcare professional and require more information for your patients, please contact us through the below.
OncoBeta is not able to provide any medical or diagnostic information.