Information for Healthcare Providers

Download the guidelines for referring clinicians
Includes overview of treatment indications and patient suitability, method of action, efficacy, safety and patient reported outcomes, and relevant clinical references.
Please provide your details to receive our clinical guideline booklet
Rhenium-SCT®
How does it work?
Rhenium-SCT® utilises the beta particles emitted during the decay of the radioactive isotope, rhenium-188, to destroy cancer cells.2 Beta particles induce direct local cytotoxic effects, such as apoptosis, as well as an immune reaction.2
The Rhenium-SCT® (Skin Cancer Therapy) is a brachytherapy utilising the Beta emitter radioisotope Rhenium-188 for the treatment of non-melanoma skin cancer.
The main objective of the Rhenium-SCT® is to be a painless, personalised, non-invasive therapy targeting and destroying cancer cells in the area needed to treat.
Rhenium-188 is an isotope that emits ß-radiation, which has excellent therapeutic advantages.
ß-radiation is the emission of ß-particles, which can penetrate the human tissue only up to 2-3 mm deep. Therefore, rhenium-188 is ideal in treating superficial tissue without affecting other parts of the body.
It has a half-life of 17 hours, which means that the radioactivity decays by half every 17 hours, allowing treatment to be relatively quick, whilst avoiding the accumulation of radioactive waste in the clinic.
> 0 %
complete response
> 0 %
excellent or good cosmesis

Patients to consider for
Rhenium-SCT®

Rhenium-SCT® has successfully treated:2,3,5
- BCC and SCC lesions, including:
- Superficial and nodular tumours
- Infiltrative and ulcerated tumours, and
- Recurrent tumours.
Difficult-to-treat areas treatable with Rhenium-SCT® include: 2,3,5,6
- Face (including nose, ear and lip)
- Digits
- Genitals
- Locations where wound closure might be more challenging.2
- BCC and SCC lesions, including:
Rhenium-SCT® has successfully treated:2,3,5
- BCC and SCC lesions, including:
- Superficial and nodular tumours
- Infiltrative and ulcerated tumours, and
- Recurrent tumours.
Difficult-to-treat areas treatable with Rhenium-SCT® include: 2,3,5,6
- Face (including nose, ear and lip)
- Digits
- Genitals
- Locations where wound closure might be more challenging.2
- Malignant melanoma
- Skin tumours affecting nerves or bony structures
- Lesions of the upper eyelid
- Lesions which anatomical position does not allow a proper application of the Rhenium-SCT resin/paste
- Confirmed pregnancy or impossibility to rule out a pregnancy
- Illnesses which require medication that significantly suppresses wound healing or the immune system
- Patients under 18 years
- Existing major circulatory disorders in the region to be treated
Treatment process
Rhenium-SCT® (Skin Cancer Therapy) is a form of nuclear medicine that utilises the Beta emitter radioisotope rhenium-188 for the treatment non-melanoma skin cancer.
1
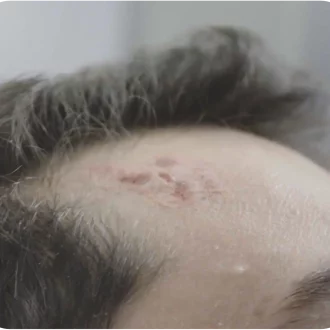
Diagnosis and Depth Determination
Following visual identification of suspicious lesions, the clinician will perform a punch or excisional biopsy to confirm diagnosis and maximum depth

2
Lesion Demarcation & Referral
If the histopathology report indicates a BCC or SCC < 3mm deep, the patient may be suitable for Rhenium-SCT if they are not good surgical candidates. If referring for a Rhenium-SCT consultation, lesion demarcation, preferably using dermoscopy, is helpful for treatment planning. The referring clinician should provide good quality clinical photography with lesion demarcation and pathology reports as part of the referral process.

3
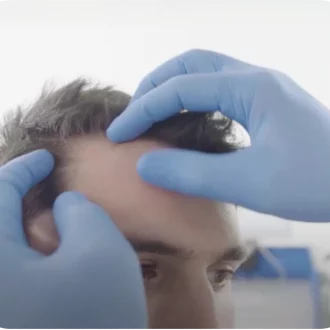
Treatment
On treatment day, the clinician will demarcate the lesion based on the report and photography provided by the referring skin specialist, to ensure precise application of the resin. The rhenium resin is applied onto adhesive film affixed to the lesion, thereby avoiding direct contamination of the skin with the radioactive resin. Precise treatment time is calculated using the activity applied and the biopsy-determined lesion depth – the range is usually 30-180 minutes. The treatment is painless, and the patient sits comfortably until the treatment time elapses.
4
Aftercare & Followup
Once the resin is removed, the patient can immediately return to regular activities. They will not be radioactive. The treating clinician and nurse will provide them with aftercare advice, however, there is no complex procedures apart from keep the area clean. Patients will be informed of expected treatment reactions and timings. The area will become inflamed with crusting over the following weeks, which is a normal part of the radiodermatitis skin reaction. A follow-up visit will be planned with the treating clinic. The patient may also return to the referring clinician as part of their ongoing skin cancer surveillance and care. Further information is available on request for referring clinicians.
Rhenium-SCT® Application Suite
Engineered to ensure safety, convenience and quality control.

Healthcare Provider Enquiries
Healthcare providers can use this form to contact us for additional information or support.
Patient or Carer?
For general enquiries, please use the patient enquiry form and your request will be forwarded to a treatment centre for follow up.